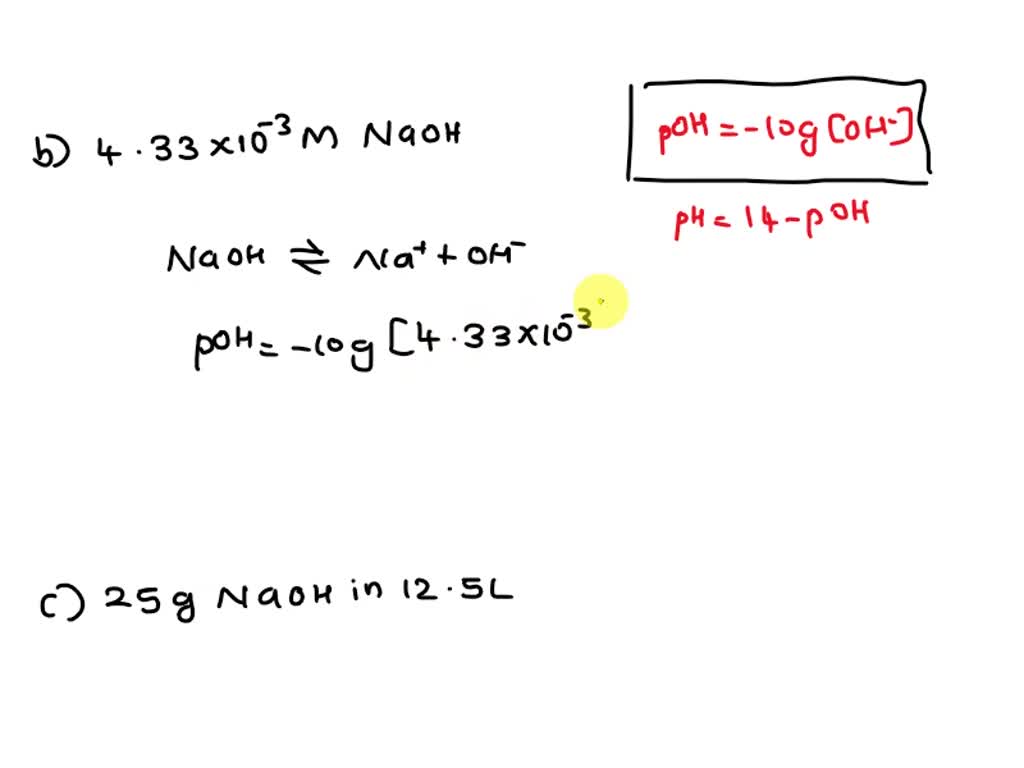
SOLVED: 7) What are the pH values of the following solutions? a) A 1.45 x 10-5 M HCl solution. b) A 4.33 x 10-3 M NaOH solution. c) A solution that contains

Assertion : `pH` of `10^(-7) M HCl` is less than `7` at `25^(@)C`. Reason : At very low concentratio - YouTube
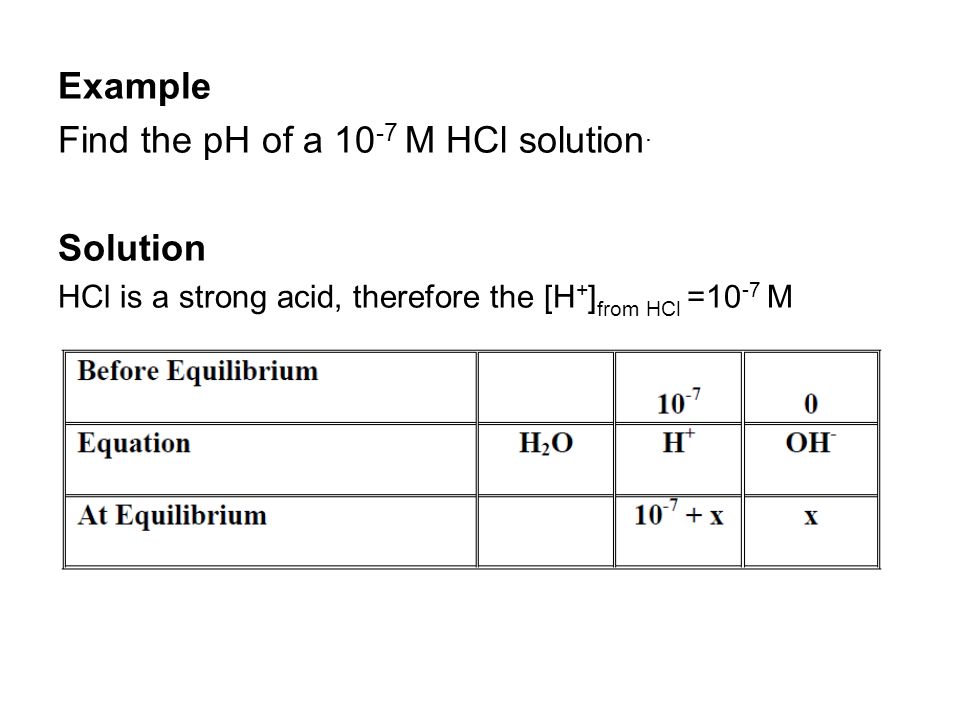
We are faced with different types of solutions that we should know how to calculate the pH or pOH for. These include calculation of pH for 1. Strong acids. - ppt video
31 0.1 ml 0.001 M hcl solution is diluted with water to make 10 litres .calculate PH of the dilute solution.

SOLVED: What is the pH of a 2.0 x 10^-7 M HCl solution? Hint: consider the background H3O+. pH = 6.61 pH = 6.70 pH = 6.79 pH = 6.52
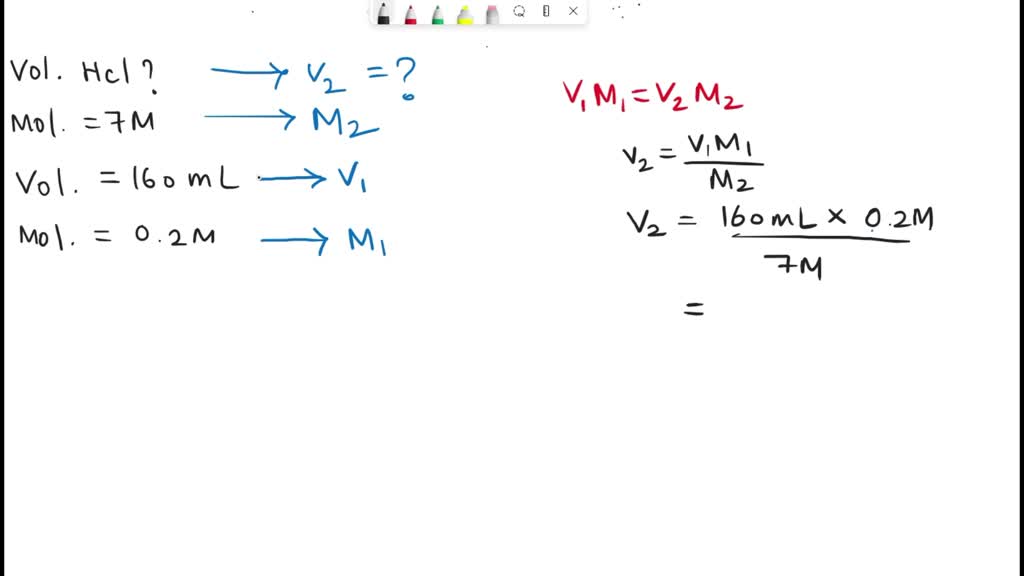
SOLVED: 2. How many ml of 7 M HCL solution is required to prepare 150 mL of 0.2 M HCL solution? Calculate the pH of the resulting dilute acid solution.


![pH of 10−6MHC( aq. )s:YH2O→[H+]=10−7M= pH of 10−6MHC( aq. )s:YH2O→[H+]=10−7M=](https://classroom-images.cdn.askfilo.com/classroom/1643793759519_ngsxesns_354069.jpg)

![Solved Calculate the pH of each solution. a) [H30+] = 1.7 x | Chegg.com Solved Calculate the pH of each solution. a) [H30+] = 1.7 x | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F0fa%2F0fa98cc3-4673-4562-9448-02d65a91573c%2Fimage)


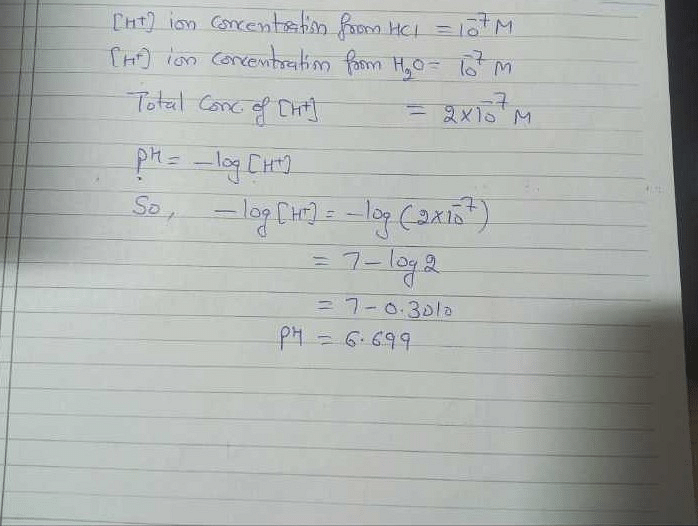


![Malayalam] The pH of a 10^(-7) M aq. solution of HCl at 298 K is 7 Malayalam] The pH of a 10^(-7) M aq. solution of HCl at 298 K is 7](https://static.doubtnut.com/ss/web/9899943.webp)
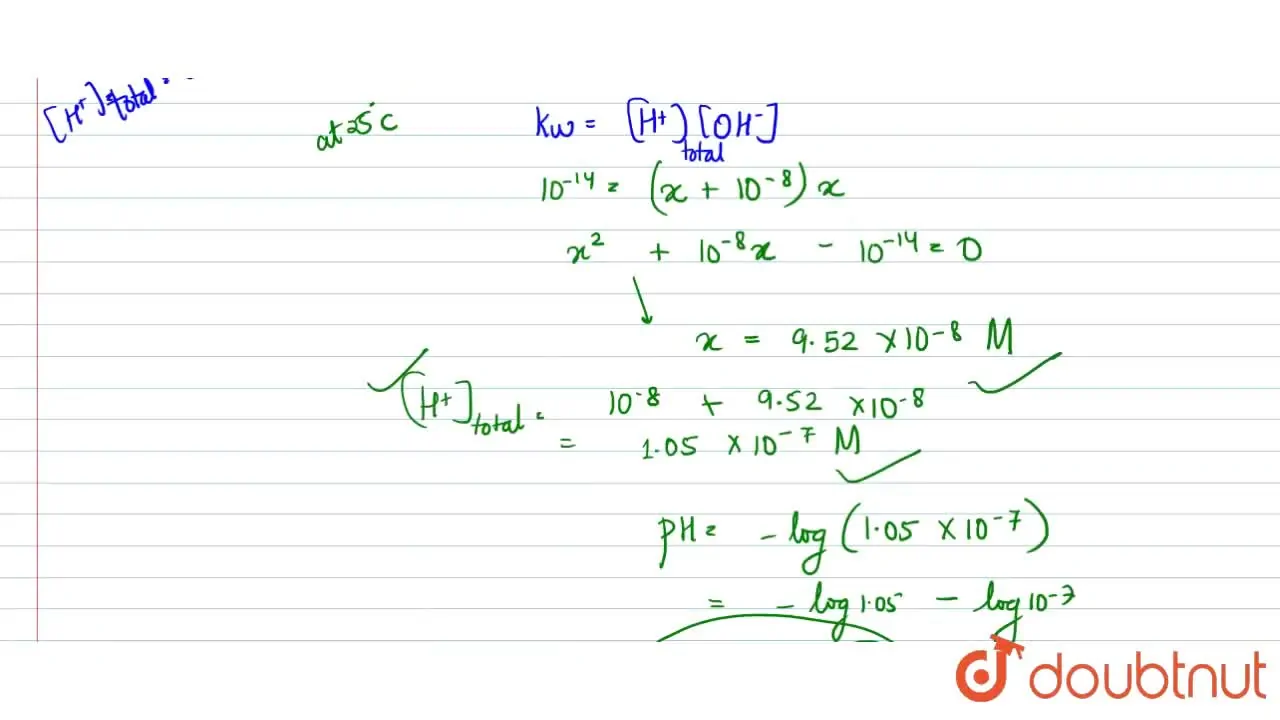
:max_bytes(150000):strip_icc()/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)