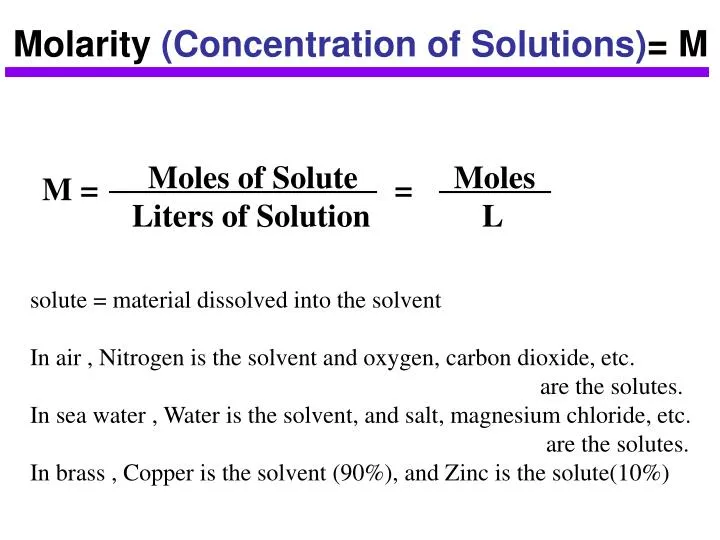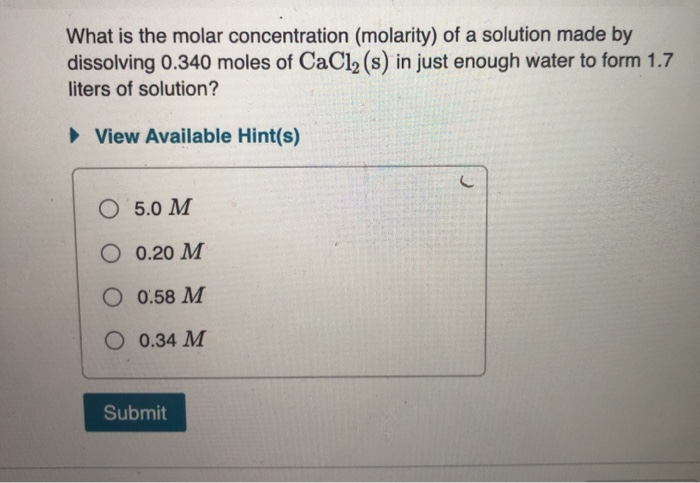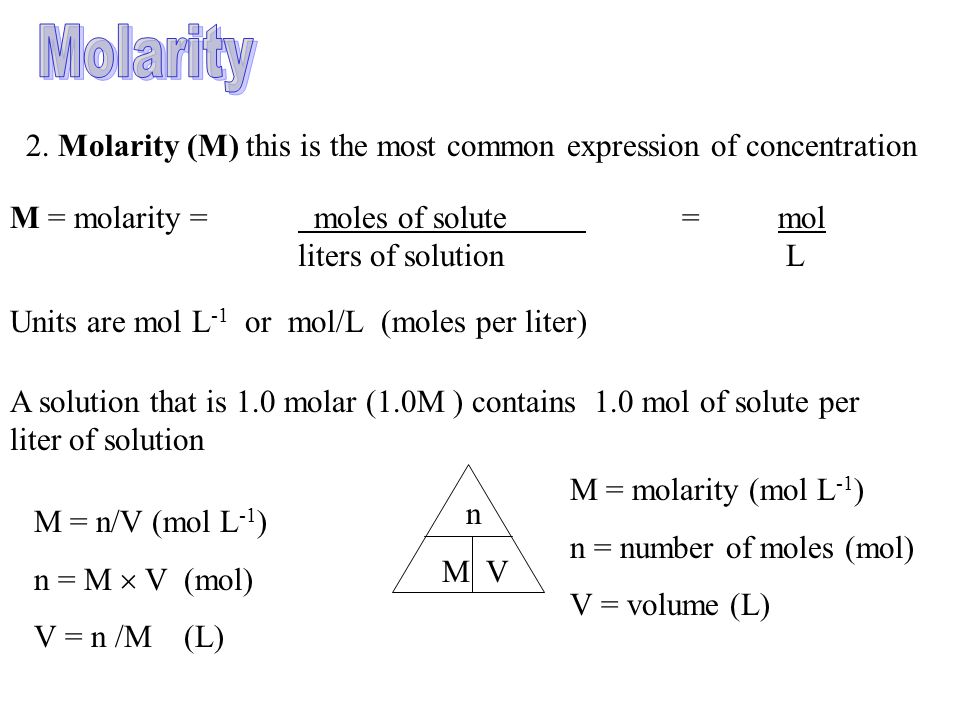
Molarity 2. Molarity (M) this is the most common expression of concentration M = molarity = moles of solute = mol liters of solution L Units are. - ppt download
How many grams of glucose, C6H12O6, are necessary to prepare 598 ml of solution with concentration of 0.72 molar? - Quora

Molarity . . Molar concentration (also called molarity, amount concentration, or substance concentration) is a measure of the concentrati... | Instagram

Calculating molarity units molar concentration of solutions practice questions on molarity how to make up a standard solution how to determine solubility of a salt gcse chemistry igcse KS4 science A level

SOLVED: Determine the molar concentration of the dye in the stock dye solution that you used, as follows: (show work for credit) Read the absorbance value (AO) at the peak of the

Molar concentration of Ag+ ion in the mixture of 50ml of 0.1M AgNO3 and 50 ml of 0.2M NaCl is nearly (Ksp of AgCl=10-10M2) 1) 2x10-M 2) 2x10-8 M 3) 0.05M 4) 5x10-10 M